Abstract
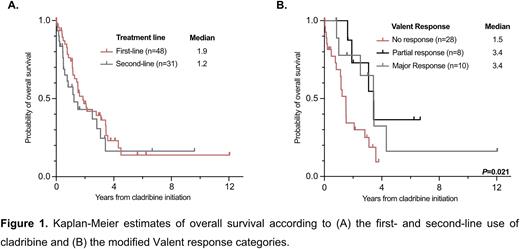
For nearly two decades, the off-label treatment with the purine analogue cladribine was considered as the standard-of-care for patients with advanced systemic mastocytosis (AdvSM), which is driven by the KIT D816V mutation in >90% of patients. High response rates and improved progression-free- and overall survival (OS) have favored the use of the multikinase inhibitor midostaurin and the specific KIT D816V inhibitor avapritinib, but cladribine remains a relevant treatment option beyond first-line (1L) treatment due to intolerance, resistance and progression on KIT inhibitors. Based on data from the 'German Registry on Disorders of Eosinophils and Mast Cells (GREM)', we therefore sought to evaluate response and resistance to cladribine in both 1L- and second-line (2L) in 79 AdvSM patients. Prior treatment included midostaurin while subsequent treatment approaches included individually or sequentially midostaurin, avapritinib, acute myeloid leukemia-like intensive chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT). Treatment options with a low disease-modifying impact (e.g. interferon-alpha) or solely directed towards the associated hematologic neoplasm (e.g. hydroxyurea, azacytidine) were not considered as 1L- or 2L-treatment. This study is an updated and more detailed analysis of a recently published comparative study between midostaurin and cladribine (Lübke et al., J Clin Oncol 2022). Cladribine was used at a dose of 0.14 mg/kg/day subcutaneously or intravenously on days 1-5 of a 28-day course. For 1L- (n=48, 61%) and 2L-treatment (n=31, 39%), a median number of 3 cycles was applied over a median of 3.3 (range 0.1-16.0) and 3.0 (range 0.1-28.5) months, respectively (P=0.612). Three or more cycles were applied in 32/79 (41%) patients (1L, n=21, 66%; 2L, n=11, 34%). The main reason for dose reduction, e.g. application only on days 1-3 or extension of intervals, was prolonged myelosuppression (15/79, 19%). Separating 1L- and 2L-treatment, the overall response rate (ORR), according to modified Valent criteria (46 evaluable patients), was 41% (12/29) and 35% (6/17, P=0.690), and median OS (all patients evaluable) was 1.9 years (48/79), and 1.2 years (31/79), respectively (P=0.311). Independent of the treatment line, application of ≥3 vs. <3 cycles (median OS 2.8 vs. 1.2 years, P=0.038) and response vs. no response after at least 1 cycle (median OS 3.4 vs. 1.5 years, P=0.002) correlated with improved OS. After minimizing confounding effects through treatment with midostaurin, avapritinib, intensive chemotherapy or allogeneic HSCT, multivariable analyses revealed leukocytosis ≥16 x 109/L (hazard ratio [HR] 5.0, 95% confidence interval [CI 1.2-21.0], P=0.026) and eosinophilia ≥1.5 x 109/L (HR 3.0 [CI 1.0-8.8], P=0.048) as independent adverse prognostic markers for OS. There was no impact of other laboratory (anemia, thrombocytopenia, serum tryptase; P>0.517) or genetic markers (mutations in SRSF2, ASXL1 or RUNX1; P=0.412), on OS. Due to an excess mortality of low- and intermediate-risk patients, none of the recently established prognostic scoring systems (MARS, IPSM, MAPS or GPSM) were predictive for OS. In conclusion, cladribine is effective in 1L- and 2L-treatment of AdvSM. The presence of leukocytosis or eosinophilia, application of <3 cycles and a lack of response according to modified Valent criteria are adverse prognostic markers. The various prognostic models for AdvSM are of limited value because of high mortality in low- and intermediate-risk patients. Overall, cladribine remains a relevant sequential treatment option for patients with AdvSM.
Disclosures
Kreil:Incyte: Research Funding. Horny:Novartis: Consultancy; Blueprint Medicines Corporation: Consultancy. Sotlar:Novartis: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Smart-in-Media: Consultancy, Honoraria. Cross:Astellas Pharma: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Valent:Blueprint Medicines: Honoraria; Celgene: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Incyte: Honoraria. Schwaab:Novartis: Consultancy, Honoraria; Blueprint Medicines: Consultancy. Reiter:AOP: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
OffLabel Disclosure:
Use of the purine analogue cladribine in advanced systemic mastocytosis
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal